Lithium ion battery refers to a secondary battery composed of two compounds that can reversibly insert and extract lithium ions as the positive and negative electrodes. People call this kind of lithium ion battery with a unique mechanism that relies on the transfer of lithium ions between the positive and negative electrodes to complete the battery charging and discharging work.
“Rocking chair battery”, commonly known as “lithium battery”.
Lithium batteries usually have two appearances: cylindrical and square. The inside of the battery adopts a spiral wound structure, and a very fine and highly permeable polyethylene film separator is used to separate the positive and negative electrodes. The positive electrode includes a current collector composed of lithium cobalt oxide (or lithium nickel cobalt manganate, lithium manganate, lithium iron phosphate, etc.) and aluminum foil. The negative electrode is composed of a current collector composed of graphitized carbon material and copper foil. The battery is filled with an organic electrolyte solution. In addition, a safety valve and PTC components (partial cylindrical use) are also installed to protect the battery from damage when the battery is abnormal and the output is short-circuited.
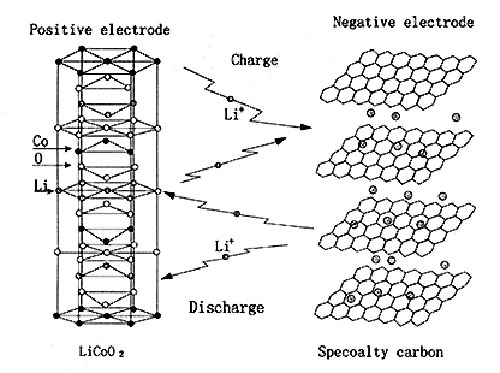
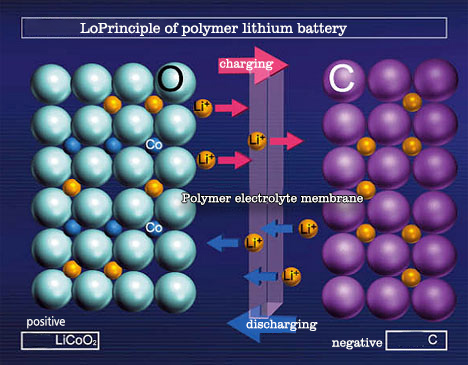
The voltage of a single-cell lithium battery is 3.7V (3.2V for lithium iron phosphate anode), and the battery capacity cannot be infinite. Therefore, single-cell lithium batteries are often processed in series and parallel to meet the requirements of different occasions.


